
Nuclear medical imaging
By Frank Deconinck
Among the may applications of nuclear energy
and ionising radiation, medical imaging certainly is least subject
to negative perception or outright opposition from the general
public. Proponents of nuclear power correctly refer to it as an
example of a very positive use of nuclear technology. Working
in the field of medical imaging, it appeared to me that some misunderstandings
or confusions exist as to the principles behind the different
medical imaging techniques and their potential diagnostic role.
This paper, which is a shorter version of an article entitled
'Nuclear Imaging in the Realm of Medical Imaging' (Nuclear Instruments
and Methods in Physics Research A 509 (2003) 213–228), gives
a general introduction to the subject.
1. The spectrum of medical imaging techniques
Medical imaging techniques can be classified
according to a number of criteria. A particular classification
scheme could use appearance, e.g. tomographic versus non-tomographic
images and would group CT and MRI because of the similarity in
image presentation. Another would classify the techniques according
to the underlying physics. This is the classification scheme which
is used here. Its basis will be the origin and nature of the radiation
source that will carry the information about the patient to a
detector.
1.1 External sources
When the radiation source is external, the body
structures modulate the information through interactions with
the radiation. In X-ray radiography or CT, an external point source
of X-rays is used. The X-rays are partially absorbed when the
rays pass through the body. The rays that are neither absorbed
nor scattered move in straight lines between the point source
and the detector (e.g. film), thus creating a shadow image of
the bodily structures.
In ultrasound, an external source of pulsed
sound waves is used. Both the time and direction of the pulse
is known. Interfaces between different tissues will partially
reflect the sound waves. By measuring the time span between the
outgoing and incoming sound pulse, images can be reconstructed.
In endoscopy, an external light source illuminates
internal organs through glass fibre. An ocular or small camera
is used to observe the reflected light and hence the organ.
1.2 Internal sources
The body naturally and continuously radiates
heat: its source is internal. In order to image the information
carriers, some optics for infrared radiation are needed: a thermographic
camera.
In MRI, the information is carried by radio
waves emitted by hydrogen nuclei in the body. Although the body
cxontains plenty of hydrogen nuclei, e.g. in water molecules,
the nuclei do not naturally emit radio waves. In order for them
to do so, they have first to be put in a magnetic field and then
activated by means of well chosen radio wave pulses at specific
frequencies. The nuclei then 'answer' by emitting radio waves
of similar frequencies. In MRI the internal sources are always
present but they only emit information when activated to do so.
In nuclear imaging, the information is carried
by gamma rays emitted by internal radioactive tracers. The body
is naturally radioactive. For physical reasons due to the nature
of the radioactive decay of the radioactive body constituents,
imaging them is too difficult to be of any use. Also, from the
medical point of view, the information would not be of much help.
Therefore, artificial radioactive tracers are administered. They
are chosen in such a way that their radioactive decay allows for
external detection and that their space/time distribution reflects
clinical information.
Because of their particular importance, ultrasound, MRI, radiography
and nuclear imaging will be discussed in more detail.
2 Ultrasound
The medical use of ultrasound is a spin-off from
Japanese research on sonar. The first US scanners became available
in the early fifties and the technique entered widespread clinical
practice in the seventies.
The information in ultrasound originates from
the reflection of sound waves emitted by an external source, typically
a piezoelectric crystal resonance of between 1 and 10 MHz. Refraction,
absorption and scattering also play a role, but mainly as factors
that degrade the clinical information. The basic physical parameters
of importance are the frequency of the wave, the speed of sound
v and the density ? of the tissue.
The reflected fraction at a muscle/fat interface
is about 1%. At a skin/air interface the reflected fraction becomes
99.9%, hence the use of a gel to decrease this undesired reflection.
Among many others, there are two typical artefacts
in ultrasound. The first artefact is due to the coherent nature
of the sound wave: the sound wave is a coherent pulse which will
interfere with its reflected, refracted and transmitted components
to give rise to speckling, similar to the speckling observed in
laser light. The second artefact is due to the physics of reflection:
interfaces between tissues that are parallel to the wave propagation
will not reflect the wave and will therefore not be seen in ultrasound.
For some applications such as obstetrics or cardiology,
the clinical information in the images is very high. Furthermore,
the technique is safe and relatively inexpensive. Current research
tends to eliminate artefacts, improve the image contrast and improve
the presentation of the data. Many efforts are directed towards
3D or even 3D + time data acquisitions and representations.
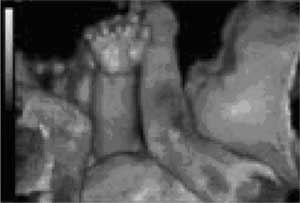
FIGURE 1: 3D ultrasound (© 2000 General Electric,
www.gemedicalsystems.com)
3 Magnetic Resonance Imaging
The MRI technique stems from physics research
carried out by Gorter, Rabi, Purcell, Bloch and many others that
led to the discovery and development of nuclear magnetic resonance
techniques just before and after world-war II. Medical applications
and imaging were introduced in the seventies by, among others,
Lauterbur, Damadian and Mansfield.
The basic information in MRI imaging relates
to
-
the magnetisation of hydrogen nuclei (their magnetic moment
is called 'spin'), denoted as N(H)
-
the energy transfer between the spins and tissue, characterised
by a time constant T1
-
the energy redistribution among spins with a time constant
T2
-
flow
Without an external magnetic field, the magnetic
moment of the hydrogen nuclei will point at random in all directions.
There will be no net magnetisation. In a large external magnetic
field, the hydrogen nuclei in tissue will preferentially align
their spin (1/2 or –1/2 due to quantum mechanical laws)
along the magnetic field. More spins will align their spin in
the direction of the field ('spin-up') than in the opposite direction
('spin-down') because the energy in spin-up direction is lower
than in spin-down direction. The global energy of the spin system
will, therefore, decrease while the magnetisation increases.
This magnetisation implies a transfer of energy
from the spin system to another system: the 'lattice', or tissue
in the case of MRI. This transfer of energy is characterised by
an exponential relaxation law with a time constant T1,
also called spin-lattice relaxation time. In typical MRI field
strengths (0.5 to 1.5 T), T1 is typically of the order
of 0.5 to 2 s, depending on the tissue type.
Next to interacting with the lattice, the spins
can also interact among each other: as one spin flips from down
to up, another spin can absorb the released energy and flip from
up to down. This spin-spin redistribution of energy, internal
to the spin system, is also characterised by a relaxation time,
called spin-spin relaxation time and noted as T2. Typical
values for T 2 are 10 – 100 ms, again depending
on the tissue type.
For a typical MRI field strength of 1.5 T the
energy difference spin up/down corresponds to radio waves with
a resonant frequency of 60 MHz.
By sending radio waves at resonant frequency
some spins which were spin-up will absorb the energy of the wave
and flip to spin-down, thereby increasing the global energy of
the spin system. The energy of the spin system will now no longer
be in equilibrium with respect to the tissue temperature and hence
violate the normal Boltzmann distribution in equilibrium. The
spin system will subsequently re-emit the extra energy as radio
waves at resonant frequency. By varying local magnetic fields
('gradients'), fine-tuning the frequency, the polarisation and
the duration of radio wave pulses to excite the spin system, and
by modulating the delay after which the re-emitted waves (the
'signal') are measured, MRI images can be reconstructed. The contrast
in the images then depends on the four following factors: N(H),
T1, T2 and flow (any movement of nuclei during the imaging sequences).
The clinical value of MRI images is recognised
in a large number of pathologies. Examples are the base of the
skull and articulations such as the knee.
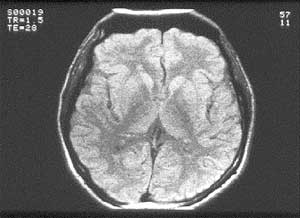
FIGURE 2: MRI image (1983) with Fourier reconstruction
artefact (bottom folded to top).
Current research tends to widen the scope of
information gathered. Examples are magnetic resonance angiography
(MRA) to visualise the vascular structure without injection of
contrast media, functional MRI to visualise areas of specific
brain function, and diffusion imaging. Other ongoing efforts involve
the shortening of the acquisition times that used to be tens of
minutes and are now between seconds and a few minutes.
MRI is a rather safe technique for both patients
and staff. Obvious precautions, such as removing metallic objects
that could fly into the magnet due to the very high field strength
should be taken. Patients with internal metallic objects such
as clips should be excluded from the imaging procedure. The same
is true for patients with pacemakers. Most other potential hazards
are associated with the generation of heat due to induced currents.
4 Radiography
Radiography is imaging with an external X-ray
source. X-rays were accidentally discovered but not recognised
as such by Goodspeed at the University of Pennsylvania in 1890.
It is only after Röntgen's discovery in 1895 that radiography
was born. Only weeks after the discovery, medical applications
started as illustrated by figure 3.
The imaging process in radiography is based on
the detection by film or other adequate detectors of the transmission
of X-rays originating in a point source (the X-ray tube). Along
their path from source to detector, the X-rays (photons with a
mean energy in the range between 15 and 60 keV) undergo photon-matter
interactions. Among the four classical interactions, the photoelectric
effect, Compton scattering, coherent scattering and pair formation,
only the first two are relevant because of the energy range.
The photoelectric effect is the main photon-matter
interaction of importance in radiography; it creates the shadow
image through absorption by the body structures, and allows the
detection of the photons by the detector.
FIGURE 3: First Belgian military radiograph, April
1896.
X-ray film is still the most widely used detector.
However, the characteristics of film are such that it is not very
sensitive to X-rays. Therefore, a phosphor screen that transforms
the X-ray in visible light is put against the film - thereby drastically
increasing its sensitivity and allowing a similar decrease in
radiation exposure to the patient. Today, large field of view
semiconductor detectors gradually replace film.
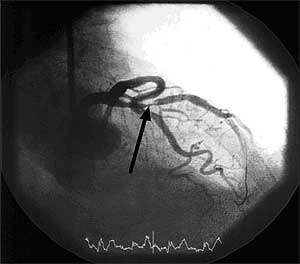
FIGURE 4: Coronarography of patient with LAD Stenosis
The spectrum of clinical applications of radiography
is overwhelming, but inherently limited by the fact that it is
a projection technique: the information along the path of the
X-ray is integrated and information on changes in absorption along
the path is lost in the image. This is the reason why X-ray computed
tomography was developed.
Because of the ionising character of X-rays,
a real health risk exists. Early radiographers paid a high toll
as victims of radiation induced illnesses such as leukaemia.
5 Computed tomography
The loss of information due to the projection
of a shadow in classical radiography limits its clinical value.
Several methods have been devised in order to overcome this loss:
tomography through blurring of out-of-focus structures by moving
the X-ray source and film in opposed directions, stereoscopic
views etc... The advent of powerful data processing allowed for
new approaches and in 1972 Hounsfield introduced Computed Tomography
(CT) following pioneering work carried out by Oldendorf and Cormack.
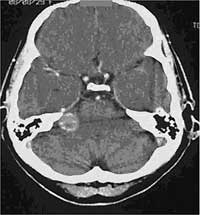
FIGURE 5: CT image with beam hardening artefacts
In order to have enough data to mathematically
reconstruct virtual slices, one needs projections from different
angles. Two angles allow the reconstruction of objects as squares.
This is of course not satisfactory. As a rule of thumb, the quality
of the reconstruction (shape, intensity...) and resolution in
an image increases with the number of projections. However, for
a fixed total acquisition time, the noise in each projection increases
also with this number. Some optimum has to be found between resolution
and noise. In today's CT scanners, thousands of fixed solid state
scintillator detectors span a 2p arc, while a X-ray tube rotates
at high speed (up to 1 revolution/s) over a full circle around
the patient. Tomographic images are then reconstructed by means
of analytical or iterative reconstruction algorithms.
As for projection radiography, a drawback of
CT is the radiation burden to the patient, especially for young
children. It is expected that the future switch from integrating
detectors to counting detectors will allow a drastic reduction
in patient dose for equivalent image quality, thus eliminating
this burden.
6 Nuclear Imaging
The use of radioactive tracers that are introduced
in the living system to study its metabolism dates from 1923 when
de Hevesy and Paneth studied the transport of radioactive lead
in plants. In 1935, de Hevesy and Chiewitz were the first to apply
the method to the study of the distribution of a radiotracer (P-32)
in rats.
The major development of nuclear imaging (also
called scintigraphic imaging) started with the invention of the
gamma camera by Anger in 1956. In parallel, positron imaging was
developed. Both imaging modalities are now standard in the major
nuclear medicine departments.
The tracer principle, which forms the basis of
nuclear imaging, is the following: a radioactive biologically
active substance is chosen in such a way that its spatial and
temporal distribution in the body reflects a particular body function
or metabolism. In order to study the distribution without disturbing
the body function, only traces of the substance are administered
to the patient.
The radiotracer decays by emitting gamma rays
or positrons (followed by annihilation gamma rays).The distribution
of the radioactive tracer is inferred from the detected gamma
rays and mapped as a function of time and/or space.
The most often used radio-nuclides are Tc-99m
in 'single photon' imaging and F-18 in 'positron' imaging.
Tc-99m is the decay daughter of Mo-99 which itself
is a fission product of U. The half-life of Tc-99m is 6h, which
is optimal for most metabolic studies but too short to allow for
shelf storage. Mo-99 has a half-life of 65h. This allows a Mo-99
generator (a 'cow') to be stored and Tc-99m to be 'milked' when
required. Tc-99m decays to Tc-99 by emitting a gamma ray with
an energy output of 14O keV. This energy is optimal for detection
by scintillator detectors. Tc-99 itself has a half-life of 211100
years and is therefore a negligible burden to the patient.
F-18 is cyclotron produced and has a half-life
of 110 minutes. It decays to stable O-18 by emitting a positron.
The positron loses its kinetic energy through Coulomb interactions
with surrounding nuclei. When it is nearly at rest, which in tissue
occurs after an average range of less than 1 mm, the probability
of a collision with an electron greatly increases and becomes
one. During the collision matter-antimatter annihilation occurs
in which the rest mass of the electron and the positron is transformed
into two gamma rays of 511 keV. The two gamma rays originate at
exactly the same time (they are “coincident”) and
leave the point of collision in almost opposite directions.
6.1 Single photon imaging
Because the source of the rays is no longer a
point source, but distributed through the object, adapted 'optics'
have to be used for image formation. There is no known material
which refracts gamma rays the way that lenses do with visible
light. One, therefore, has to rely on selective absorption of
the rays based on geometrical criteria. The first, historical
method but still used for particular applications, is based on
the 'camera obscura' principle: a lead cone is placed over the
detector and a pin-hole opening is made at top of the cone, perpendicular
to the centre of the detector surface.
Only those rays which pass through the pin-hole
form an image on the detector. The image is inverted and enlarged
or reduced with respect to the object, depending on the distances
between object, pin-hole and detector. The second method is based
on the multiple hole collimator: a thick lead or tungsten sheet
in which thousands of parallel holes are drilled (other manufacturing
techniques exist). Typical hole sizes are a couple of cm in length
with a diameter of a couple of mm. The collimator structure is
an inherent limitation to the ultimate camera resolution. Furthermore,
its geometric efficiency is very low (e.g. 10-4).
Only those rays that hit the detector through
the holes in parallel contribute to the image, which then corresponds
to a one to one mapping of the radioactive distribution.
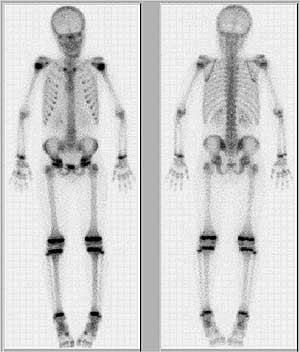
FIGURE 6: Bone scan, depicting bone metabolism in young
patient.
In the Anger gamma camera, a large (e.g. 40x60x1
cm) NaI mono-crystal is used as the scintillation detector. The
scintillations are detected by an array of about 100 photomultipliers.
The distribution among the photomultipliers of the detected scintillation
photons allows the place of detection on the crystal to be determined
with a resolution of a few millimetres. The total number of detected
photons allows their energy to be determined with a precision
between 10 and 15%: the energy resolution.
In standard nuclear medicine practice, images
are acquired during seconds to minutes. The spatial resolution
of the images is between 0.5 and 1.5 cm and the contrast resolution
is rather low. This is in part due to the fact that the images
are projection images.
Although the number of photons per pixel may
become extremely small, it may be of use to acquire series of
images to study the dynamics of large areas in the image. The
averaging effect over a large number of pixels, a 'region of interest',
then compensates for the short acquisition time. An example of
this is the use of nuclear imaging for the study of the heart
function, in which a series of 8 to 16 images, representing one
cardiac cycle, is acquired. Using specific processing techniques,
such as temporal Fourier filtering, important clinical information
can be retrieved.
By rotating the gamma camera around the patient
and acquiring a large set of projections, enough data become available
to reconstruct tomographic emission images. Kuhl developed emission
tomography in 1964.
In tomographic imaging, the spatial resolution
of the images is similar to planar imaging, but lesion contrast
and, therefore, also detectability, is greatly improved.
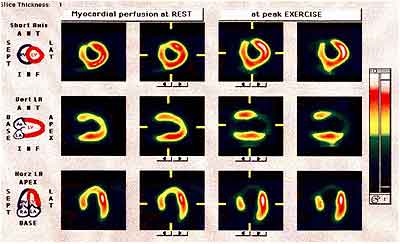
FIGURE 7: Tomographic image of myocardial perfusion
defect at exercise
6.2 Positron Emission Tomography (PET)
In PET, the administered radio-nuclide decays
due to the emission of a positron which in turn collides with
an electron and is annihilated. In the process, two 511 keV gamma
rays originate simultaneously and leave the annihilation site
in opposite directions. Positron imaging was introduced by Brownell
in 1951. Current ring PET cameras take advantage of the annihilation
characteristics. A ring of scintillation detectors surrounds the
patient. If two events are detected simultaneously in two opposed
detectors, one assumes that an annihilation occurred somewhere
on an imaginary line connecting the two detectors. By acquiring
a large number of lines, e.g. 106, tomographic reconstruction
methods can be used to reconstruct images of the tracer distribution.
The detectors used are scintillating detectors.
Their stopping power should be high enough for 511 keV photons.
Therefore, the detectors should be made out of high Z material
and have a large enough detection volume. This last point however
will reduce the precision of the localisation, as a precise spatial
localisation requires small detectors. Furthermore, scattered
rays should be rejected as they will generate lines that do not
reflect the location of the annihilation. This requires a good
energy resolution, which in turn requires large crystals. Finally,
coincident detection implies a precise timing of events. The timing
using scintillators depends on the temporal characteristics of
the light generation in the detector. Therefore, finite coincidence
time windows are set in order to accommodate for the detector
response. This inevitably will lead to 'random' coincidences,
in which two unrelated events are falsely attributed to the same
annihilation. Blurring, scattered events and random events will
therefore degrade the data sets. Current research is directed
towards improving detector characteristics, geometrical configurations
and reconstruction algorithms in order to improve the final image
quality.
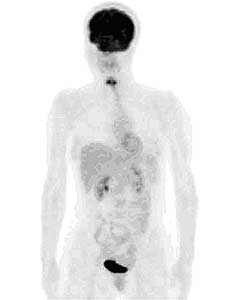
FIGURE 8: PET image of 18-FDG (DeoxyGlucose) metabolism
State-of-the-art clinical cameras have a spatial
resolution of a few millimetres, which approaches the optimum
given natural patient movements during acquisition times of the
order of minutes. Small animal scanners reach the fundamental
limit due to the positron range.
PET plays a major role in our understanding
of biological processes at the molecular level.
7 How do you choose the optimal imaging modality?
Different imaging modalities generate images
that correspond to different characteristics of the body or to
different geometrical maps. They pose different short or long-term
risks or concerns to the patient, the personnel and the working
environment. The investment and running costs of the modalities
differ, as do their availability.
The choice of an imaging technique is based
on a balanced evaluation of the above stated factors. More than
anything else, however, the following question should first be
asked and answered: If the outcome of the examination is positive
or negative, will it change the diagnostic or therapeutic pathway
for the patient? If not, the examination should not be done. |













